Spatial process or social residue?
Making sense of spatial and social heterogeneity in infection risk
InsightNet 2024
UNC Chapel Hill
2024-04-17
Jon Zelner
Dept. of Epidemiology
Center for Social Epidemiology and Population Health
University of Michigan School of Public Health
✉️ jzelner@umich.edu
🌐 epibayes.io

Michigan is an ideal place to explore the intersection of epidemiologic, health, and social systems
Not only because our state looks cool on a map.
Socioeconomically and geographically heterogeneous.
Strong statewide health data infrastructure for vital records, immunization, and reportable diseases.
Deep, long-standing collaboration between MDHHS and UM.
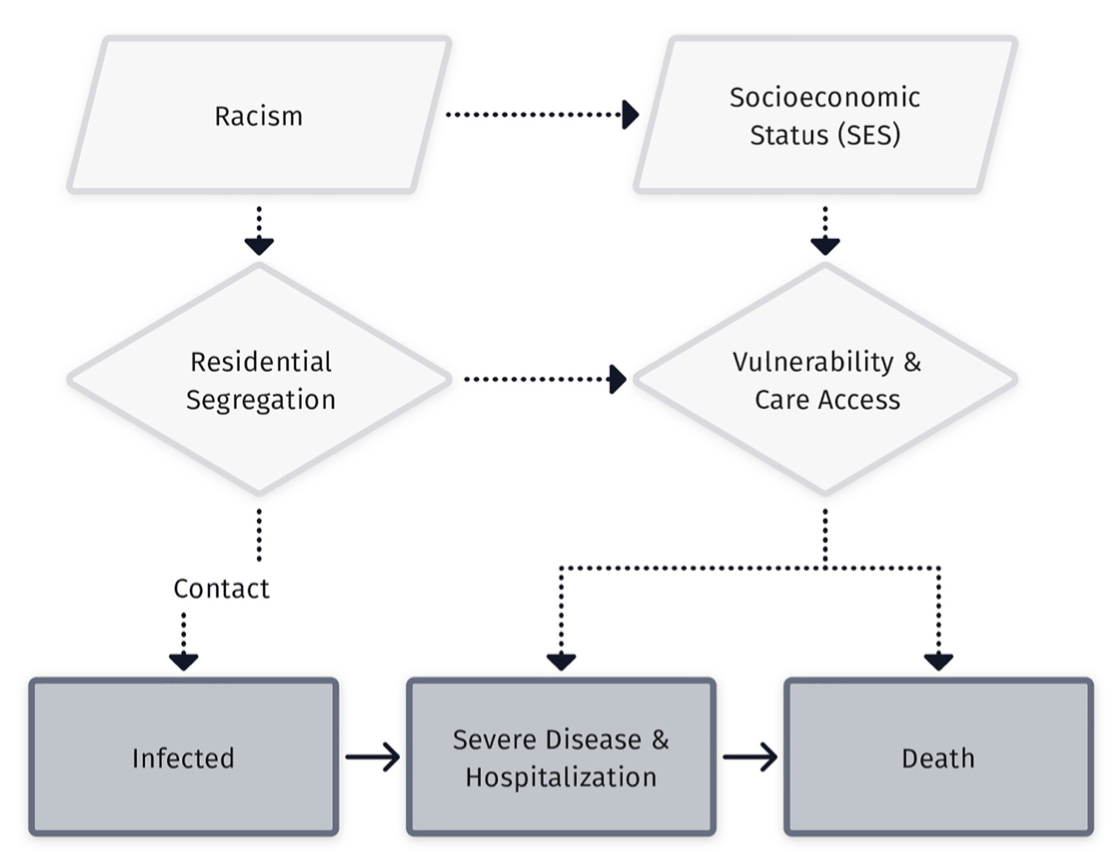
These spatial patterns reflect much more than local processes.
High-level patterns of socioeconomic inequity.
Jurisidictional differences in policy, surveillance, and intervention.
Between-community and individual-level social heterogeneity.
Demands an integrative, multi-level approach.
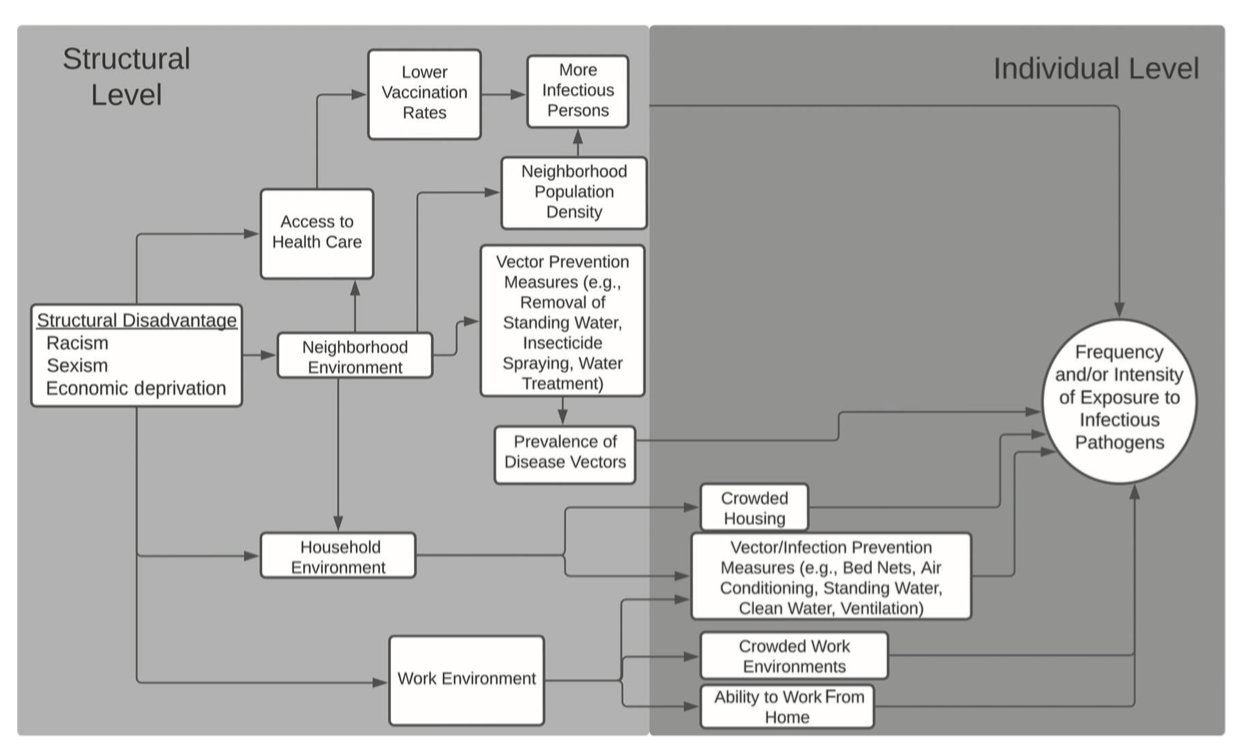
Detailed data are necessary to determine the appropriate scales of surveillance

Danger!
The appropriate scale of surveillance is not necessarily the appropriate scale for intervention.
Picking the appropriate scale of intervention requires modeling intersecting social and biologial dynamics
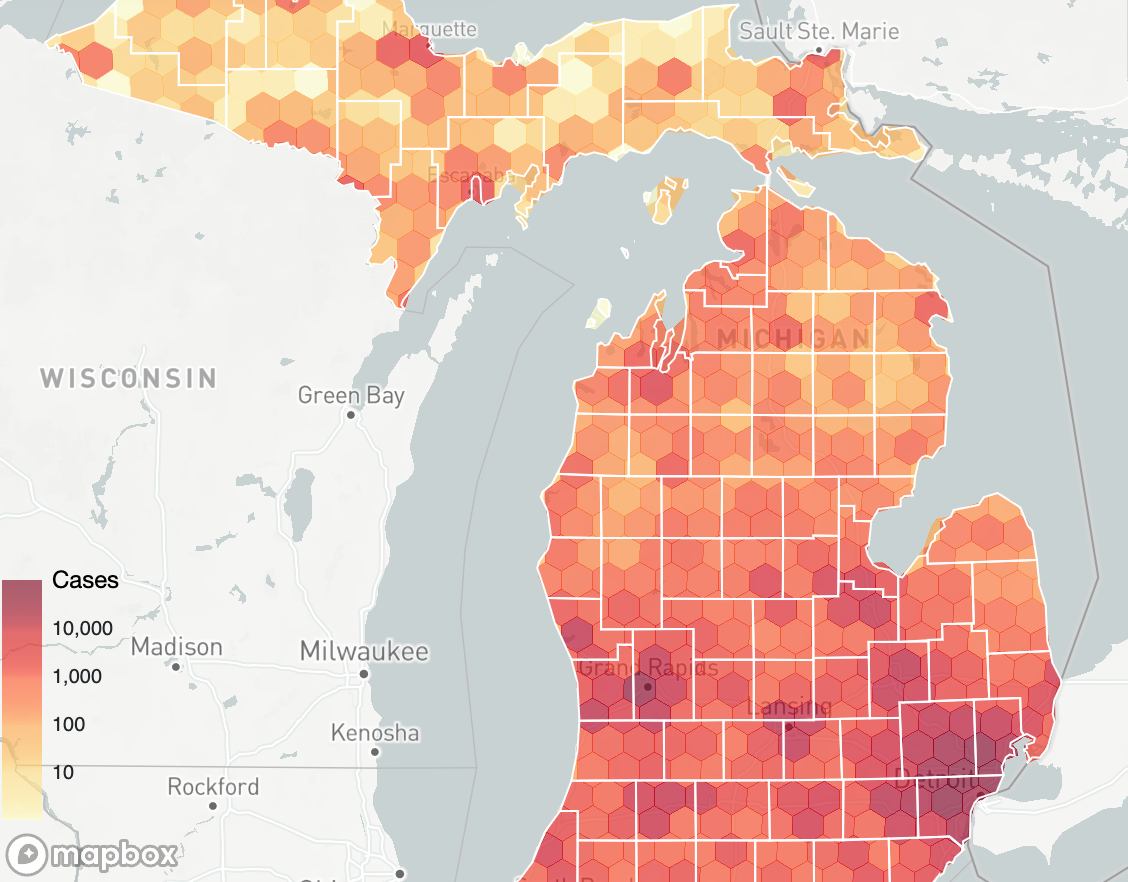
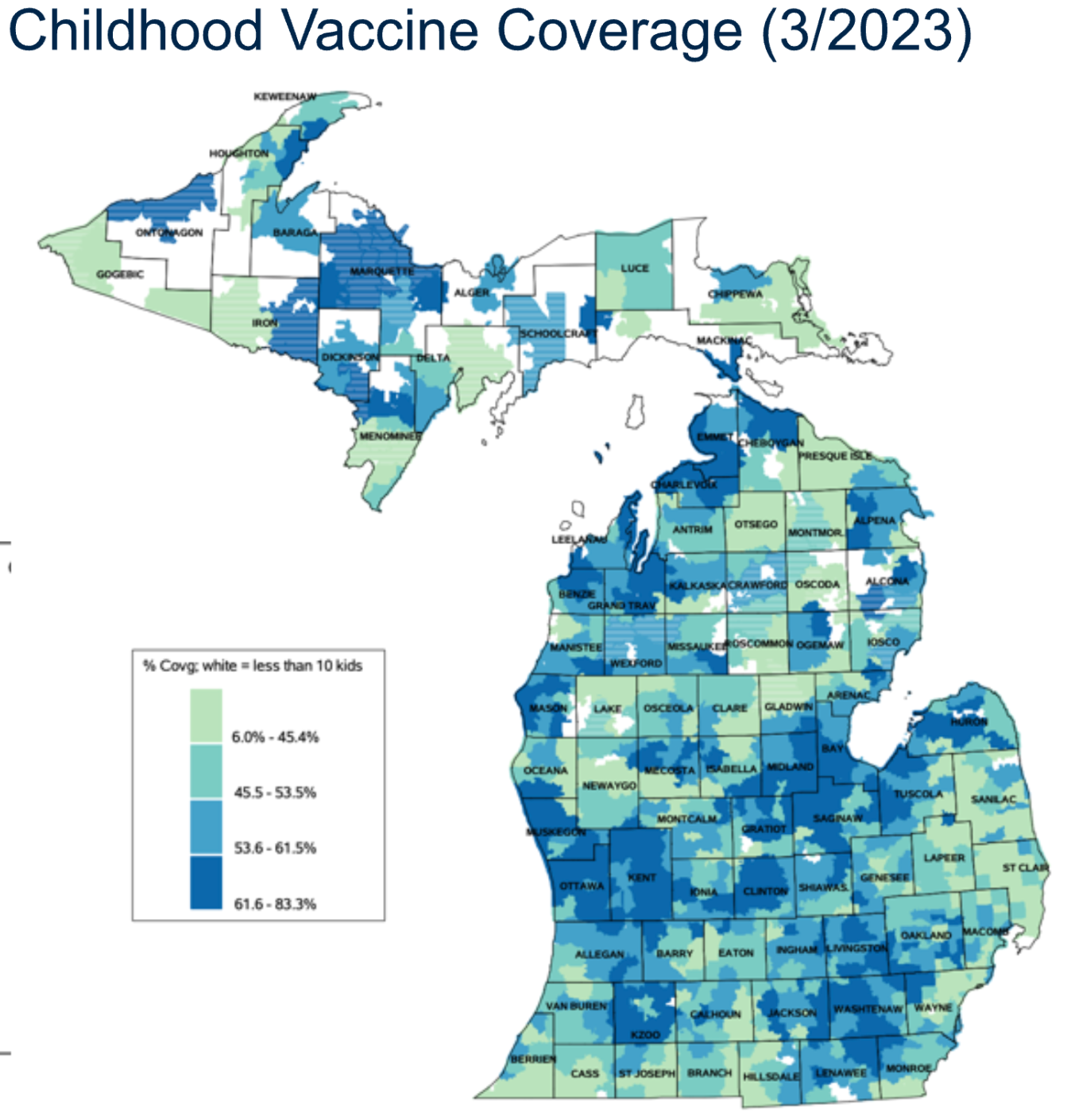
Socioeconomic variation in risk is not always visible as clusters or hotspots
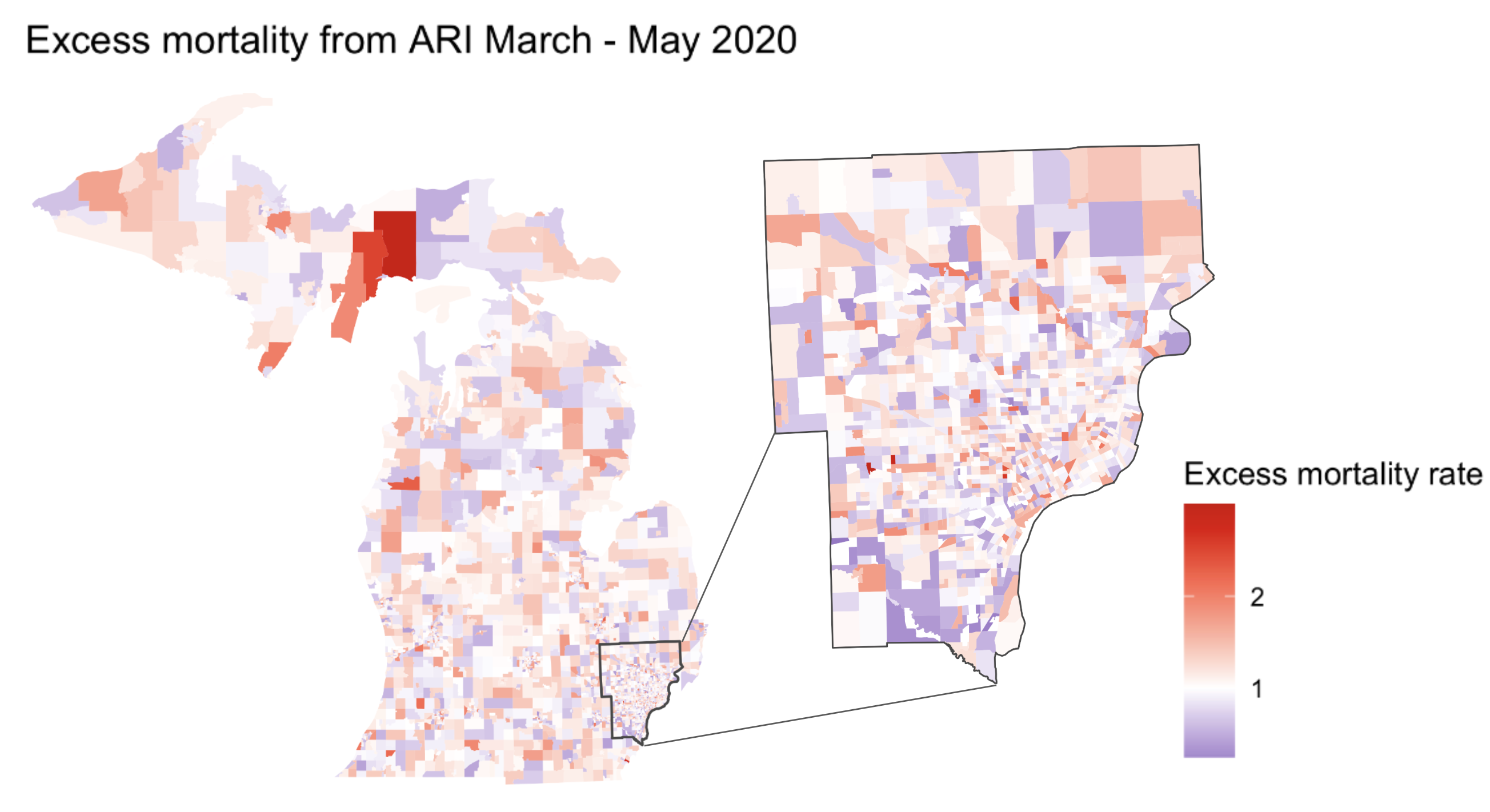
Socioeconomic inequity in excess ARI mortality in Michigan manifested largely at the sub-county level(from Herdzik et al., In Prep)
Addressing drivers of heterogeneity and inequity requires us to work across scales and systems

HAI shared-risk communities derived from Medicare patient transfer data (From Andrus et al., In Prep)
Thanks!
MDHHS, CDC partners
Kelly Herdzik, Emily Andrus
Please get in touch w/any questions etc. at
jzelner@umich.edu